Do you look for 'how to write abbreviated electron configuration'? Here you can find your answers.
Table of contents
- How to write abbreviated electron configuration in 2021
- Abbreviated ground state electrons
- Abbreviated noble gas configurations
- Abbreviated electron configuration for barium
- Abbreviated electron configuration of cobalt
- Abbreviated electron configuration of iridium
- Abbreviated electron configuration list
- Abbreviated electron configuration calculator
How to write abbreviated electron configuration in 2021
 This image illustrates how to write abbreviated electron configuration.
This image illustrates how to write abbreviated electron configuration.
Abbreviated ground state electrons
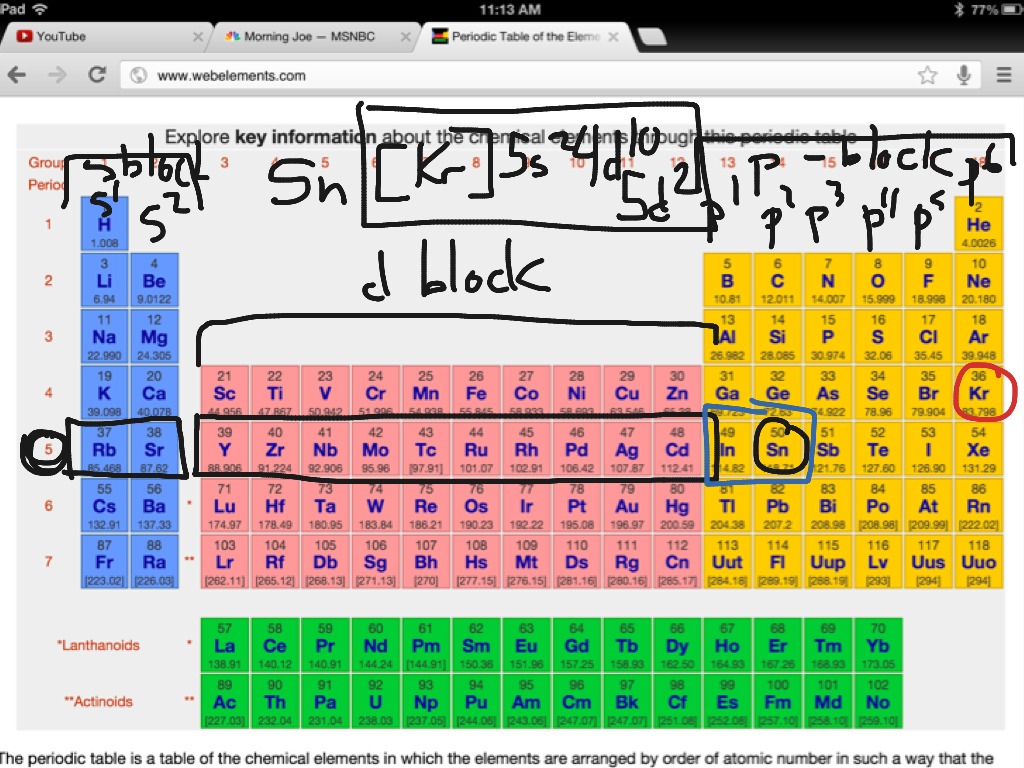 This image demonstrates Abbreviated ground state electrons.
This image demonstrates Abbreviated ground state electrons.
Abbreviated noble gas configurations
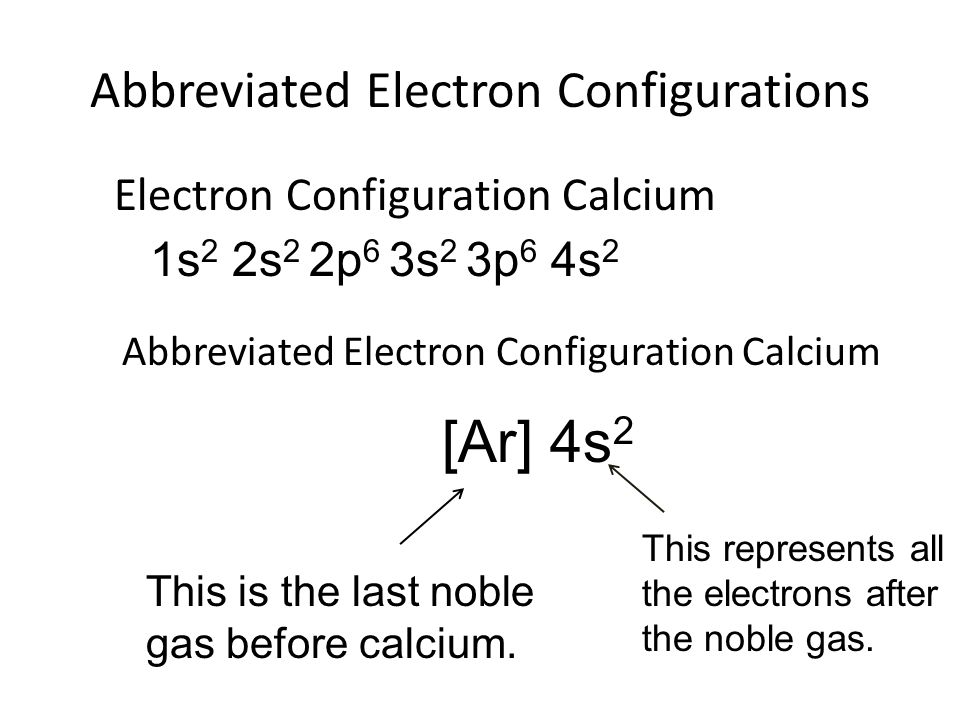 This image demonstrates Abbreviated noble gas configurations.
This image demonstrates Abbreviated noble gas configurations.
Abbreviated electron configuration for barium
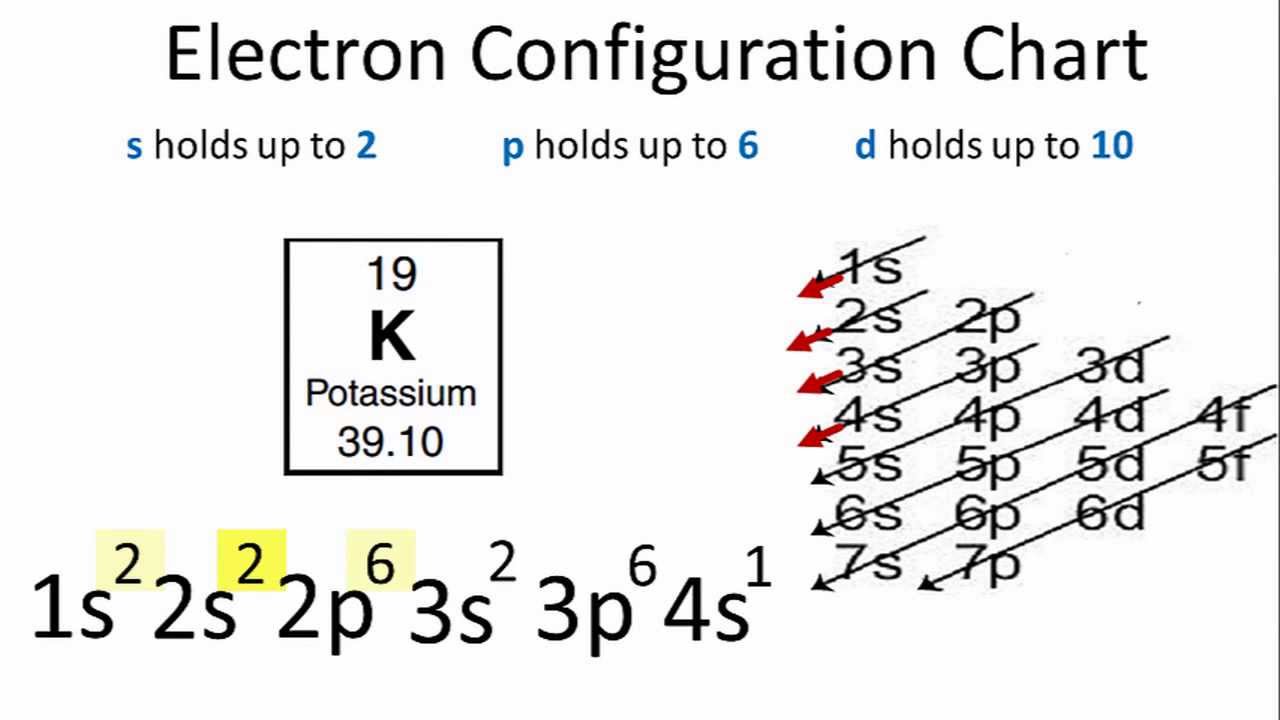 This image demonstrates Abbreviated electron configuration for barium.
This image demonstrates Abbreviated electron configuration for barium.
Abbreviated electron configuration of cobalt
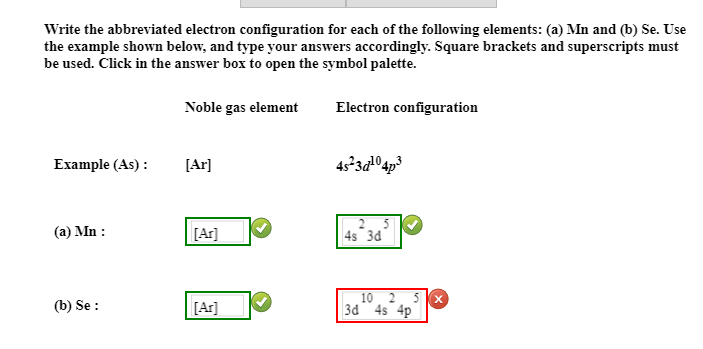 This image demonstrates Abbreviated electron configuration of cobalt.
This image demonstrates Abbreviated electron configuration of cobalt.
Abbreviated electron configuration of iridium
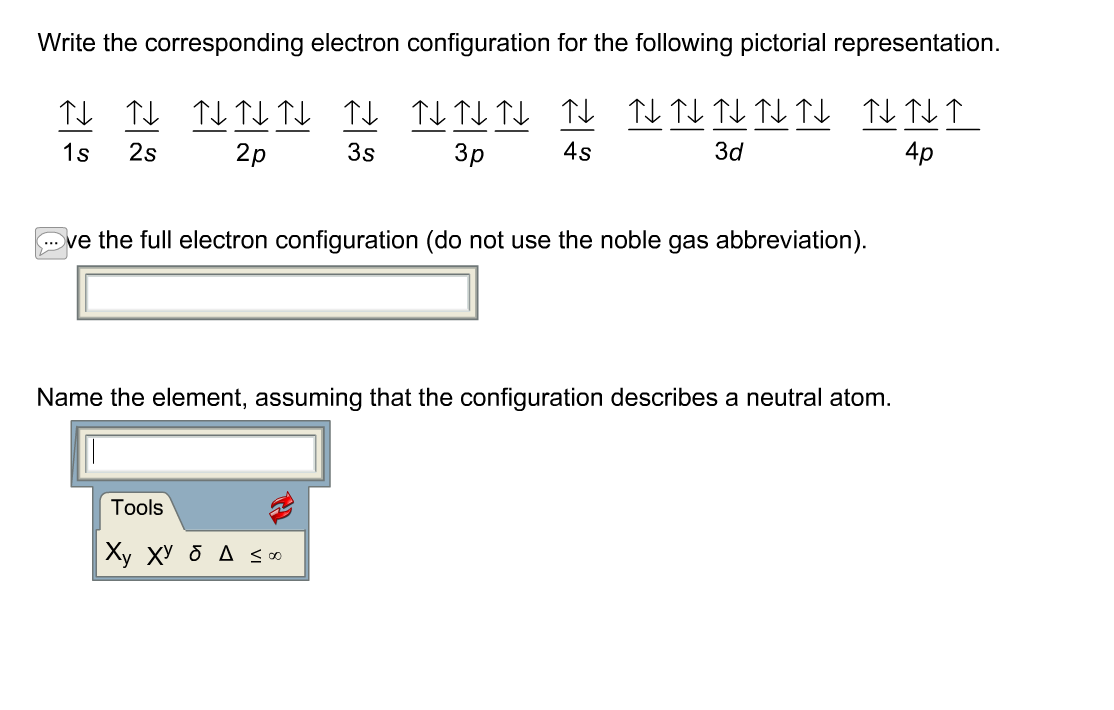 This picture demonstrates Abbreviated electron configuration of iridium.
This picture demonstrates Abbreviated electron configuration of iridium.
Abbreviated electron configuration list
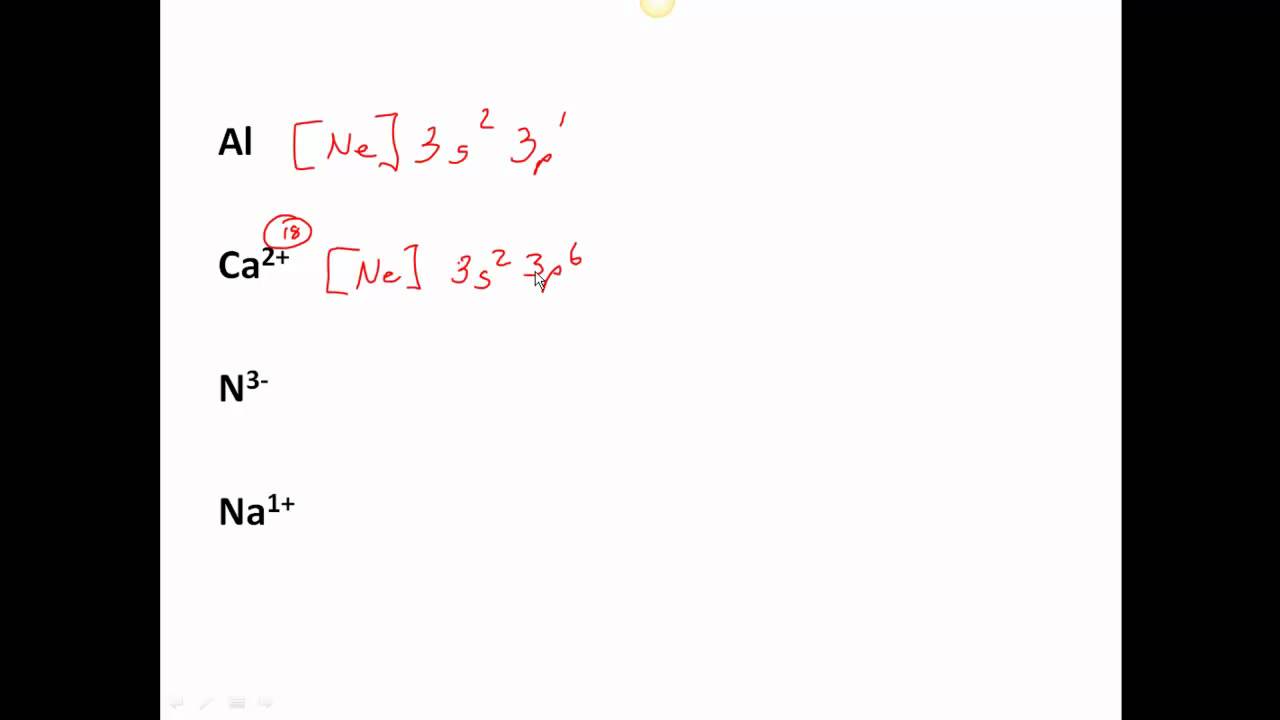 This picture demonstrates Abbreviated electron configuration list.
This picture demonstrates Abbreviated electron configuration list.
Abbreviated electron configuration calculator
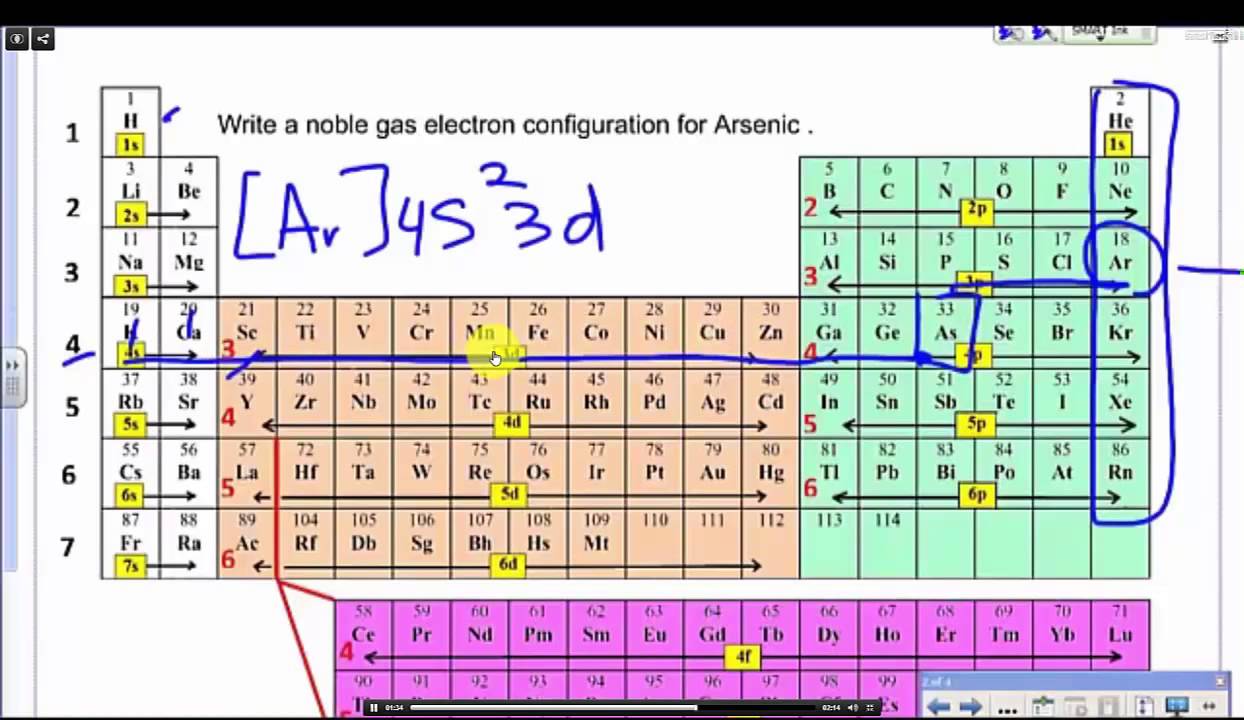 This picture demonstrates Abbreviated electron configuration calculator.
This picture demonstrates Abbreviated electron configuration calculator.
Can you give abbreviated electron configurations for elements?
PRACTICE PROBLEMS: Give abbreviated (noble gas) electron configurations for the elements below. Use this periodic table if possible. If that does not work for you try the o rbital periodic table. Click here to cancel reply.
Why do chemical scientists shorten the electron configurations?
Because some of the complete electron configurations can be so long, chemical scientists have come up with a way to shorten them. It involves using the closest noble gas above the element you are trying to give an electron configuration to. Examples: Give the abbreviated (noble gas) electron configurations for the elements below.
How to describe the electron configuration of a zinc atom?
To describe the first 18 electrons of a zinc atom, we write Step 3 Move back down a row (to the row containing the element you wish to describe) and to the far left. Following the elements in the row from left to right, write the outer-electron configuration associated with each column until you reach the element you are describing.
How are noble gases used in electron configurations?
The abbreviated electron configurations uses Noble gas configurations, which have full electron shells, to describe the electronic structure of later elements. Now we know that on the basis of these fully occupied electronic configurations, the Noble Gases are supremely unreactive.
Last Update: Oct 2021