Are you searching for 'how to write chemical formulas for ionic compounds'? Here you will find all the details.
Table of contents
- How to write chemical formulas for ionic compounds in 2021
- How to form ionic compounds
- How to do ionic compounds
- How to write chemical formulas for molecules
- Ionic compound formula list
- Ionic compound formula examples
- Writing chemical formulas practice worksheet
- List of ionic compounds
How to write chemical formulas for ionic compounds in 2021
 This image demonstrates how to write chemical formulas for ionic compounds.
This image demonstrates how to write chemical formulas for ionic compounds.
How to form ionic compounds
 This picture illustrates How to form ionic compounds.
This picture illustrates How to form ionic compounds.
How to do ionic compounds
.PNG) This image shows How to do ionic compounds.
This image shows How to do ionic compounds.
How to write chemical formulas for molecules
 This image shows How to write chemical formulas for molecules.
This image shows How to write chemical formulas for molecules.
Ionic compound formula list
.PNG) This picture demonstrates Ionic compound formula list.
This picture demonstrates Ionic compound formula list.
Ionic compound formula examples
 This image representes Ionic compound formula examples.
This image representes Ionic compound formula examples.
Writing chemical formulas practice worksheet
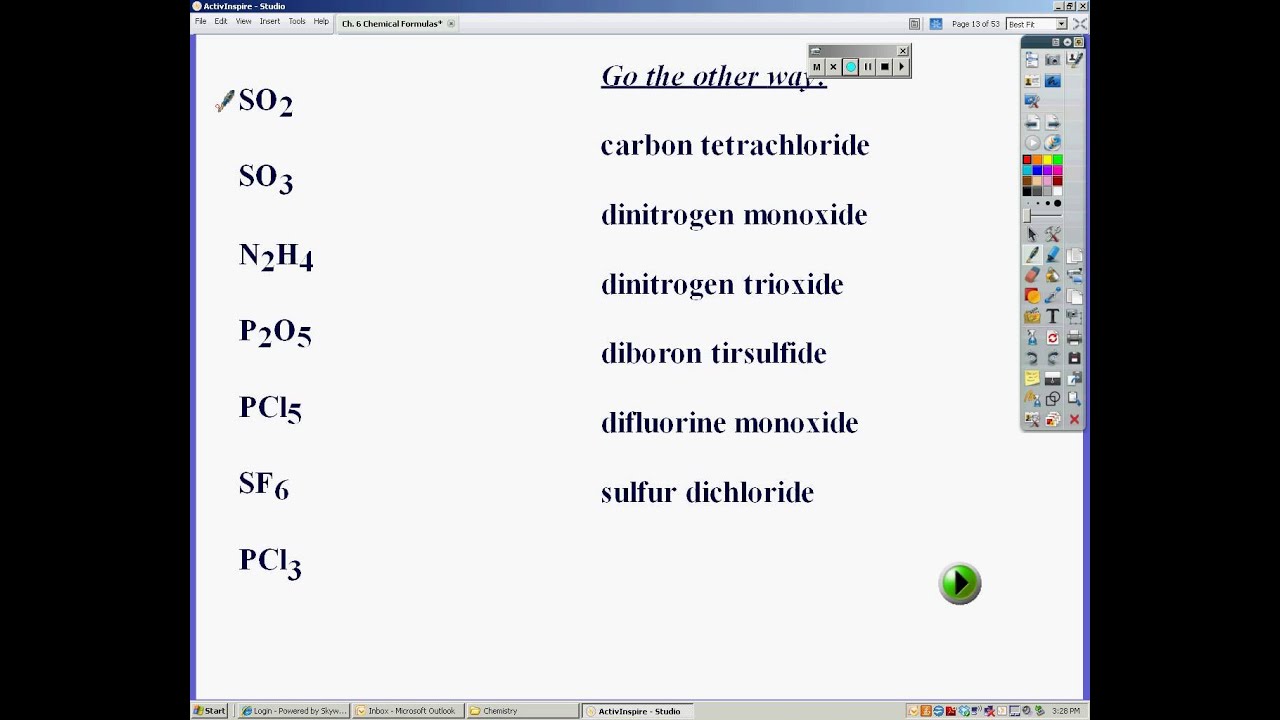 This picture representes Writing chemical formulas practice worksheet.
This picture representes Writing chemical formulas practice worksheet.
List of ionic compounds
.PNG) This picture demonstrates List of ionic compounds.
This picture demonstrates List of ionic compounds.
What is written first in the formula for ionic compound?
When writing the formula for the ionic compound, the cation comes first, followed by the anion, both with numeric subscripts to indicate the number of atoms of each. Polyatomic ions are a set of covalently bonded atoms that have an overall charge, making them an ion.
What are some simple rules for naming ionic compounds?
The Rules for Naming Ionic Compounds. Rules for naming ionic compounds: Always name the metal ion first. Name the nonmetal ion second. Change the ending of the nonmetal or second element by adding -ide. Use lower case letters for the compound name.
What type of formula is used for an ionic compound?
Chemical Formulas of Ionic Compounds Ionic compounds are composed of a metal and a nonmetal (positive and negative ions) A chemical formula shows the kinds and numbers of atoms in the ionic compound. Example: Ionic Compound: Sodium Chloride. Chemical Formula NaCl = 1 Na atom = metal (+) = 1 Cl atom = nonmetal (-)
How are chemical formulas of binary ionic compounds generally written?
Answer. Explanation: Binary ionic compounds are compounds formed by a metal and a non metal where a metal loses electron to form positively charged cation and the non metal gains electrons to form negatively charged anion. The chemical formula of ionic compound is written by writing the cation first and then the anion.
Last Update: Oct 2021
Leave a reply
Comments
Elmond
25.10.2021 10:47This means the argentiferous is written ordinal, and then the non-metal. If you acknowledge the name of the covalent pinnatifid, you can oft write the natural science formula for that compound.
Fashionette
25.10.2021 06:21Aft that is definite move to dance step number 2. Chemical formulas are written to show the ratio of elements fashionable a compound.
Margaet
27.10.2021 03:02Write out the formulas for the following geographic region compounds: naming & writing formulas of binary ionic compounds. An atom of chemical element e has 18 neutrons and letter a nucleon number of 35.