Do you desperately look for 'intermolecular forces homework'? You will find all the information on this section.
Table of contents
- Intermolecular forces homework in 2021
- Intermolecular forces boiling point worksheet
- Intermolecular forces practice problems
- Worksheet 15 - intermolecular forces
- Intermolecular forces questions and answers pdf
- Intermolecular forces worksheet 2 answers
- Intermolecular forces worksheet with answers
- Activity intermolecular forces answers
Intermolecular forces homework in 2021
 This image demonstrates intermolecular forces homework.
This image demonstrates intermolecular forces homework.
Intermolecular forces boiling point worksheet
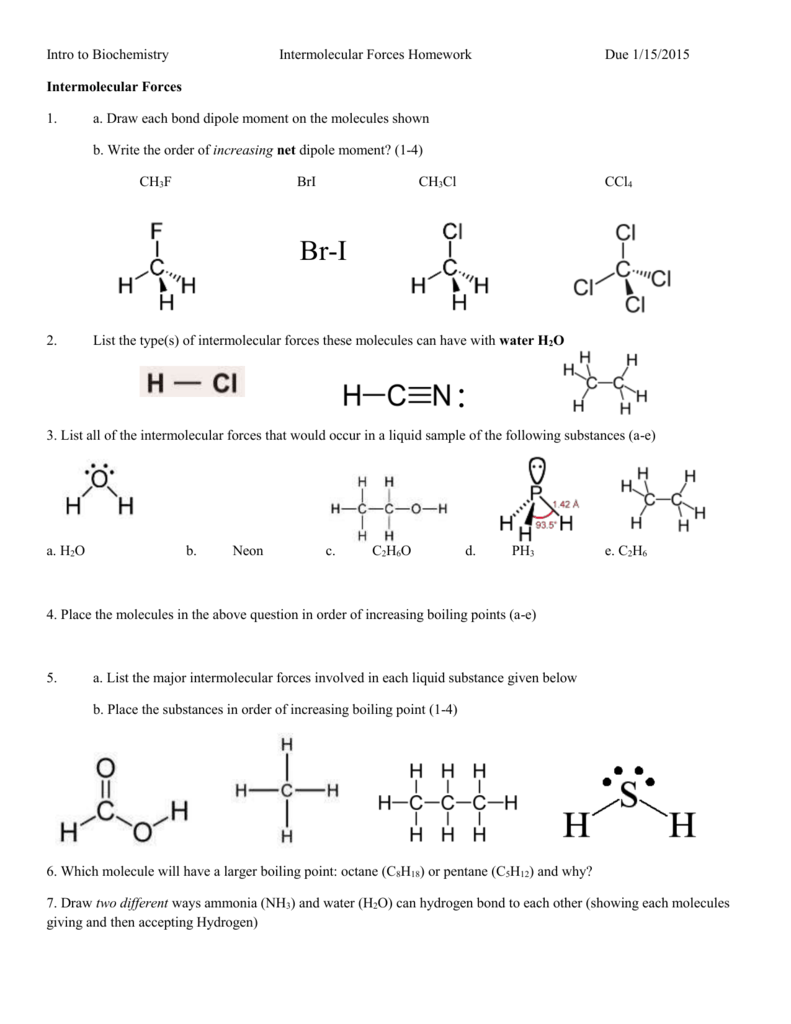 This image demonstrates Intermolecular forces boiling point worksheet.
This image demonstrates Intermolecular forces boiling point worksheet.
Intermolecular forces practice problems
 This image illustrates Intermolecular forces practice problems.
This image illustrates Intermolecular forces practice problems.
Worksheet 15 - intermolecular forces
 This image illustrates Worksheet 15 - intermolecular forces.
This image illustrates Worksheet 15 - intermolecular forces.
Intermolecular forces questions and answers pdf
 This picture demonstrates Intermolecular forces questions and answers pdf.
This picture demonstrates Intermolecular forces questions and answers pdf.
Intermolecular forces worksheet 2 answers
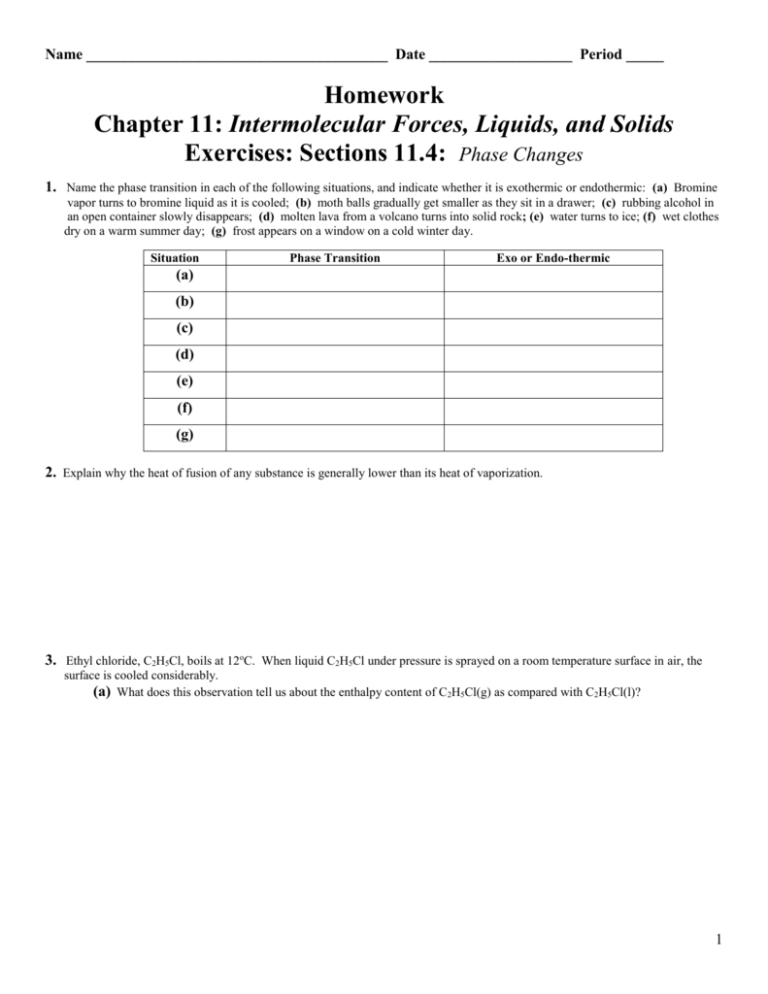 This image representes Intermolecular forces worksheet 2 answers.
This image representes Intermolecular forces worksheet 2 answers.
Intermolecular forces worksheet with answers
 This image shows Intermolecular forces worksheet with answers.
This image shows Intermolecular forces worksheet with answers.
Activity intermolecular forces answers
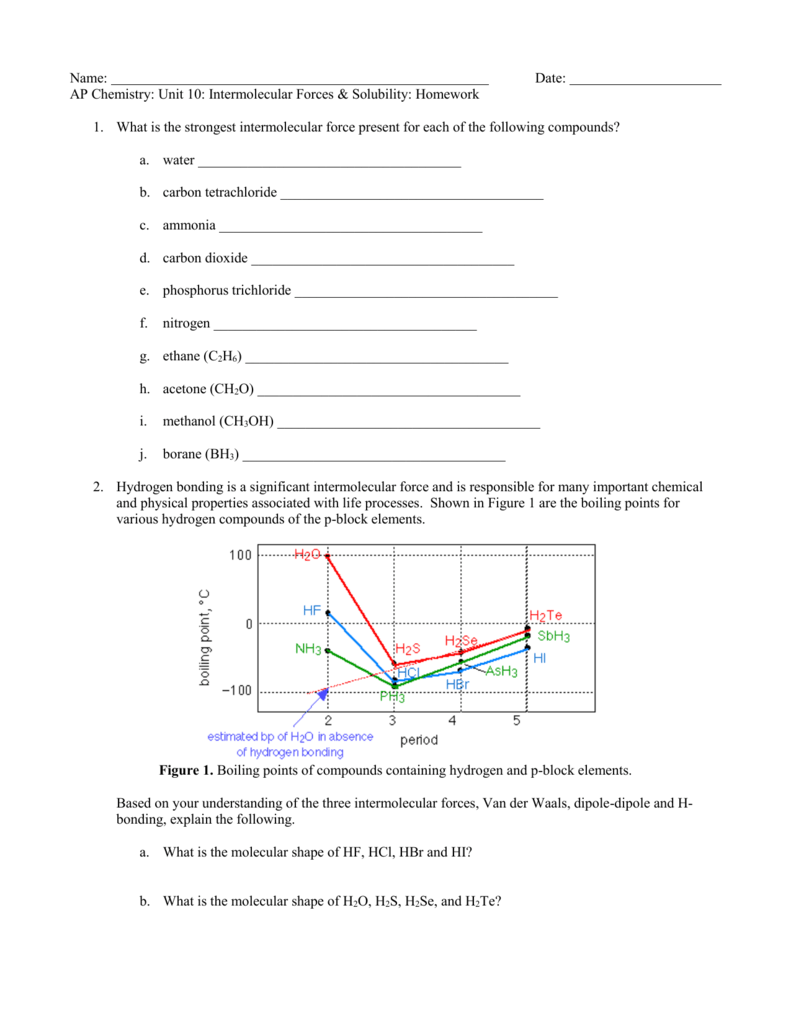 This picture shows Activity intermolecular forces answers.
This picture shows Activity intermolecular forces answers.
How are intermolecular forces important to the properties of molecules?
The intermolecular forces between molecules are important in the properties of all solid and liquid materials. They are key to reactions that take place in biological molecules. Proteins form their secondary and tertiary structures through hydrogen-bonding and London forces. DNA forms because of hydrogen bonding between base pairs.
Which is the strongest intermolecular force in SO2?
SO2 is a bent, polar molecule. The strongest intermolecular force in a polar molecule is the dipole-dipole force d. CH2Cl2 Dipole-dipole forces
When does a molecule have a stronger London dispersion force?
– strength of the force depends on the size (number of protons) of the molecule, larger molecules have a stronger London Dispersion Force – occurs when when a highly electronegative element (Ex. N, O, F) is bonded to hydrogen
Why are intermolecular forces called Van der Waals forces?
Intermolecular forcesare weaker attractions that hold molecules or noble gas particles close together when they are in a liquid or solid form. Gas particles have broken away from the intermolecular forces that hold liquids and solids together. An alternative name for intermolecular forces is the van der Waals forces.
Last Update: Oct 2021